Challenges of HIPAA Compliance For Medical Devices
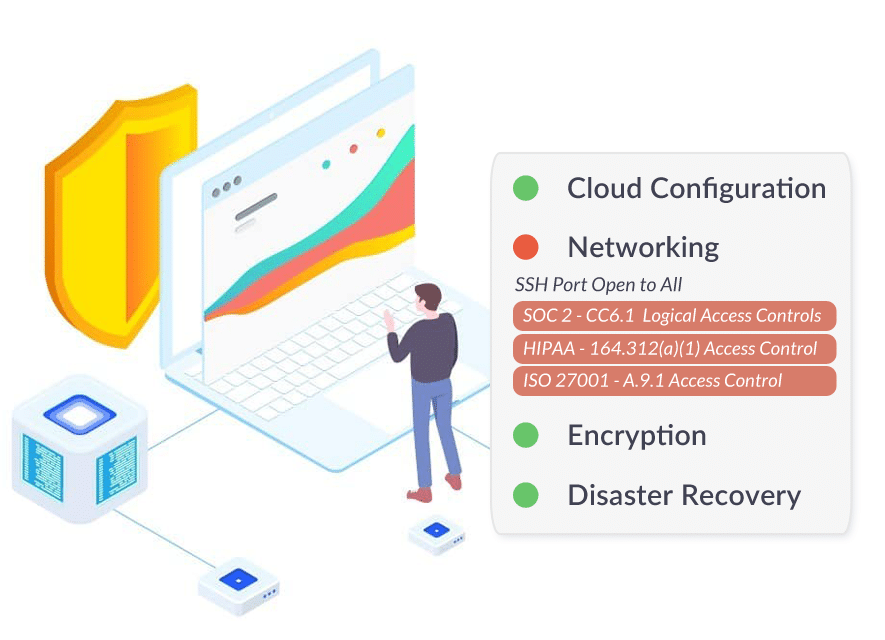
Medical Device and MedTech companies need to address HIPAA security and compliance controls when managing patient data and protected health information (PHI). As MedTech organizations prepare for clinical trials, FDA clearance, and key partnerships, it is critical that all HIPAA requirements are being met for PHI stored across infrastructure and cloud providers, as well as any PHI locally on medical devices and mobile devices.
This means that organizations need to manage administrative. technical, and physical safeguards required by HIPAA/HITECH:
- Developing HIPAA Security Program & Administrative Policies
- Handle Medical Device Security & Compliance
- Implementing Device & Transmission Encryption
- Managing Security of PHI Data In The Cloud
- Implementing Access Control Standards